Abstract
Background
VOD/SOS is a potentially life-threatening complication of HSCT conditioning, with a reported mean incidence of 13.7%. VOD/SOS with multi-organ dysfunction (MOD) may be associated with >80% mortality. Defibrotide is approved for treatment of hepatic VOD/SOS with renal/pulmonary dysfunction post-HSCT in the United States and for treating severe hepatic VOD/SOS post-HSCT in the European Union. The defibrotide expanded-access program (T-IND) was designed to provide access to defibrotide prior to its approval in the US and to collect data on safety and efficacy. There are few published data on survival of patients with neuroblastoma who develop VOD/SOS. Here, Day +100 post-HSCT survival and safety are analyzed post hoc for the subgroup of patients in that study with a primary disease of neuroblastoma.
Methods
The T-IND was the largest prospective study of defibrotide. The original protocol required VOD/SOS diagnosis by Baltimore criteria (bilirubin ≥2 mg/dL and ≥2 of: hepatomegaly, ascites, ≥5% weight gain) or biopsy, post-HSCT, with evidence of MOD. The study was amended to also include patients without MOD (off label), with VOD/SOS per modified Seattle criteria (≥2 of: bilirubin >2 mg/dL, hepatomegaly, or ≥5% weight gain [in this study]), and/or with VOD/SOS following chemotherapy without HSCT (off label). Patients received defibrotide 25 mg/kg/d in 4 divided doses (6.25 mg/kg q6h), which was recommended for ≥21 days.
Results
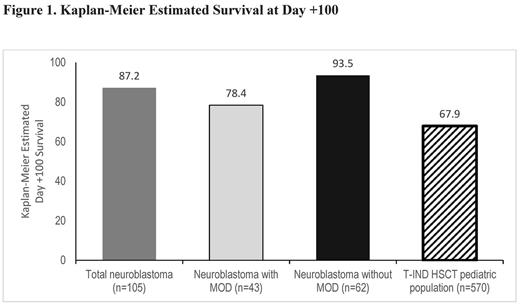
This post hoc analysis of final data is based on 1154 patients enrolled from 2007-2016 who received ≥1 dose of defibrotide, including 571 patients with MOD. The primary diseases affecting 5% or more of the safety population at baseline were acute myelogenous leukemia, 24.2% (n=279); acute lymphocytic leukemia, 24.2% (n=279); and neuroblastoma, 9.6% (n=111). Among patients with neuroblastoma, 106 had received HSCT and 5 had chemotherapy without HSCT. In the post-HSCT subgroup, 64 (60.4%) were male and 42 (39.6%) were female, median age was 3 years (range 1-17 years), with 7.6% aged 0-23 months, 90.6% aged 2-11 years, 0.9% aged 12-16 years, and 1 (0.9%) patient was aged >16 years at HSCT. At Day +100 post-HSCT, survival data were available for 105 of the 106 neuroblastoma patients treated with defibrotide with documented VOD/SOS, 43 with MOD and 62 without MOD. Of these patients, 103 had autologous transplants and 2 had allogeneic transplants. Kaplan-Meier estimated Day +100 survival for the total neuroblastoma group was 87.2% (95% confidence interval [CI], 79.0%-92.4%; Fig). For the MOD subgroup, Kaplan-Meier estimated Day +100 survival was 78.4% (95% CI, 62.6%-88.2%), and for the subgroup without MOD, 93.5% (95% CI, 83.6%-97.5%). For comparison, in the subgroup aged ≤16 years of the overall HSCT population (n=570; 127 autologous), Kaplan-Meier estimated Day +100 survival was 67.9% (95% CI, 63.8%-71.6%; Fig;Richardson PG et al. European Hematology Association 2017. Abstract P748).
Adverse events (AEs) occurred in 45.3% (48/106) of neuroblastoma patients, with serious AEs in 23.6% (25/106; most common: multi-organ failure, 4.7% [5/106]). AEs leading to discontinuation developed in 17.0% (18/106) (most common: pulmonary hemorrhage, 2.8% [3/106]); death occurred in 10.4% (11/106) (>2%: multi-organ failure, 4.7% [5/106]; veno-occlusive disease, 2.8% [3/106]). Treatment-related AEs, as assessed by the investigators and of any severity, occurred in 17.0% (18/106) of neuroblastoma patients (most common: pulmonary hemorrhage, 2.8% [3/106]).
Conclusions
There are few data on VOD/SOS outcomes in patients with neuroblastoma. This post hoc analysis of final data from the defibrotide expanded-access study found Kaplan-Meier estimated Day +100 survival of 87.2% in the comparatively large group of patients with a primary disease of neuroblastoma and VOD/SOS following HSCT (primarily autologous). Day +100 Kaplan-Meier estimated survival rates were 78.4% and 93.5% in the neuroblastoma groups with and without MOD, respectively. The Day +100 safety profile for the neuroblastoma subgroup in the expanded-access program was consistent with prior defibrotide studies and analyses of the overall HSCT population in this study. These findings provide supportive evidence for, and warrant additional studies into, the clinical utility of defibrotide for treatment of VOD/SOS in patients receiving HSCT for this common pediatric solid tumor.
Support: Jazz Pharmaceuticals.
Grupp: University of Pennsylvania: Patents & Royalties; Adaptimmune: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Other: grant. Ryan: Celator/Jazz: Employment, Equity Ownership. Hume: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Tappe: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Richardson: Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal